I want to dilute a perfumery material, how do I decide which solvent to use?
I want to dilute a perfumery material, how do I decide which solvent to use?
How-to and Guides | 4 years ago
I want to dilute a perfumery material, how do I decide which solvent to use?
It isn't an exact science but there are some things to help guide the choice of solvent. First, the vast majority of materials will dissolve well in ethanol (though there are exceptions), however ethanol has two disadvantages: it's very volatile and can gradually evaporate, leaving you with a stronger solution than you originally made, which can make formulation difficult. Also it's highly flammable which makes solutions made with it potentially dangerous to store and transport.
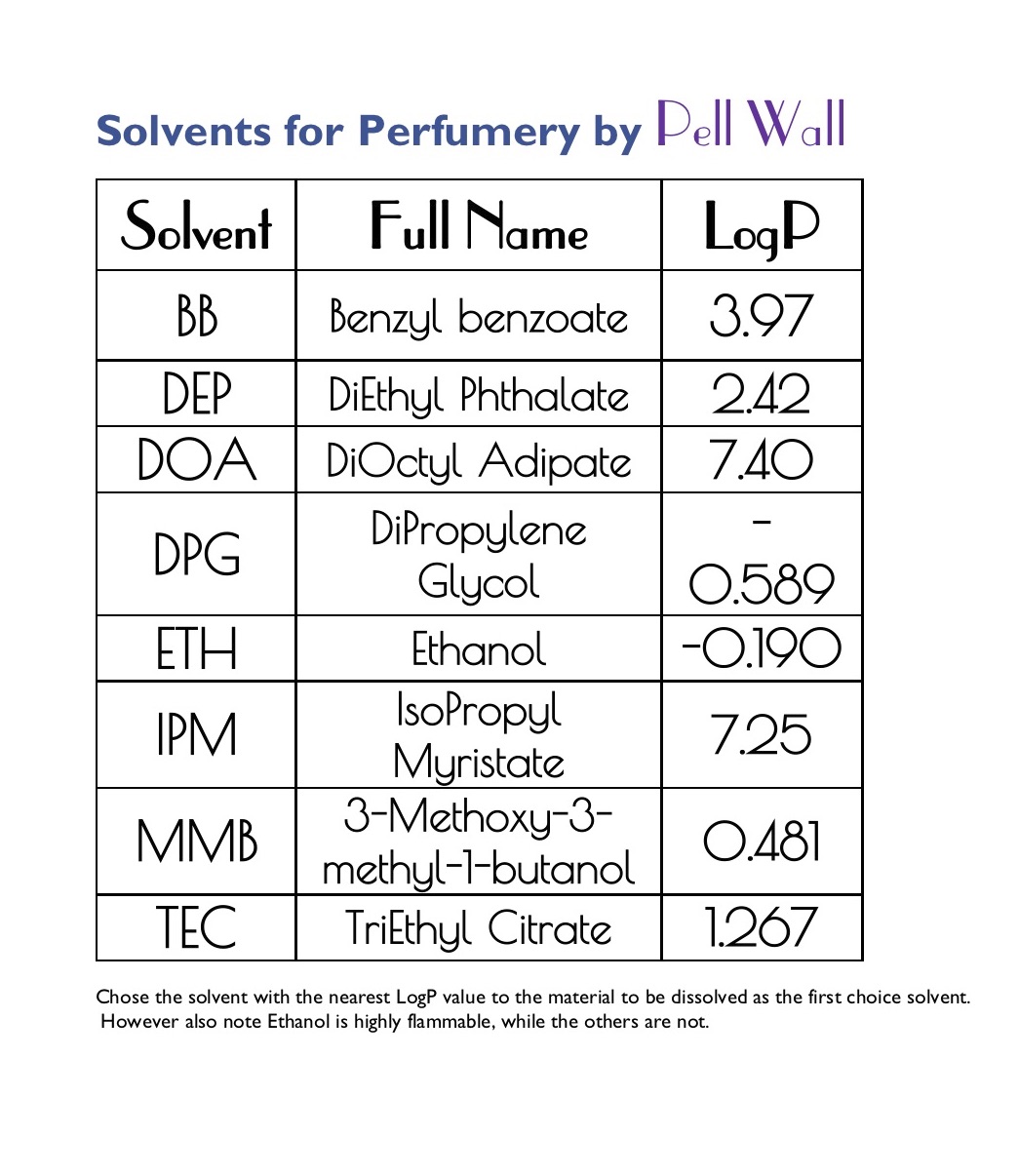
To choose an alternative solvent what you want is a solvent with a LogP (or xLogP) value that’s as close as possible to the value for the material you're going to dilute.
You can look up the LogP value on The Good Scents Company website for most materials - it’s a measure of how polar or non-polar a material is - it’s not foolproof but does give a good indication.
The attached table shows the values for the main solvents: At Pell Wall we keep printed copies around in the Lab to save looking them up every time.